One of the most significant challenges facing eye and vision researchers is the development of an effective treatment for dry age-related macular degeneration (AMD). Although there are now a number of well-regarded FDA-approved drug treatments for wet AMD, the key to effective dry AMD treatment remains elusive thus far, although several promising treatments have entered clinical trials.
Current treatments for dry AMD include a number of non-drug-related measures, including (a) nutritional supplements recommended by the Age-Related Eye Disease Study 2 (AREDS2), and (b) controlling a range of lifestyle factors, including diet, weight, blood pressure, smoking, and ultraviolet light exposure.
Stem Cell Treatments and Dry AMD
This month, information about two preliminary – but promising – stem cell treatments for dry AMD has been released to the public.
1. Advanced Cell Technology: Human Embryonic Stem Cells
On October 14, 2014, Advanced Cell Technology, Inc. announced positive results from its small (18-patient) early-stage clinical trials of human embryonic stem cells (hESC) for the treatment of dry AMD and Stargardt disease.
The research uses retinal pigment epithelial (RPE) cells derived from human embryonic stem cells. These cells are created by taking stem cells from a days-old embryo created in a fertility clinic and inducing the embryonic cells to differentiate into more specialized cells, such as RPE cells.
[Editor’s note: The deepest cells of the retina comprise the retinal pigment epithelium (RPE). The RPE helps to maintain the health of the retinal photoreceptor cells, called rods and cones. These photoreceptor cells are triggered by light to set off a series of electrical and chemical reactions that helps brain to interpret what the eye sees. The degeneration of the RPE cells also leads to the death of the rods and cones and, ultimately, vision.]
Although the primary goal of this small early-stage clinical trial was to assess the safety of the transplanted stem cells, the treatment also had unexpected positive benefits: Ten out of 18 study participants reported improvements in vision, with some subjects reporting dramatic improvements. In addition, the treatment appears to have halted the progression of the disease in 17 of the 18 subjects.
You can read more about the ACT clinical trial at Positive Stem Cell Clinical Trial Results for Stargardt Disease and Dry Macular Degeneration.
2. Cellular Dynamics International: Induced Pluripotent (Adult-Derived) Stem Cells
On Monday, October 27, Cellular Dynamics International (CDI) announced that the National Eye Institute (NEI) awarded the company a $1.2 million contract to manufacture clinically compatible induced pluripotent stem cells (iPSCs) and iPSC-derived human retinal pigment epithelial (RPE) cells.
These cells will be manufactured from individuals with dry AMD and are intended to be used for Investigational New Drug (IND) studies. If CDI’s Investigational New Drug Application is approved by the United States Food and Drug Administration (FDA), the procedure will be used to generate iPSC-derived RPE tissue for transplantation back into the same patients. This process, known as autologous cellular therapy (i.e., involving one individual as both donor and recipient) would be the first of its kind permitted in the United States.
Current Retinal Stem Cell Research in the United States and Japan
On November 22, 2010, the U.S. Food and Drug Administration (FDA) lifted a prior clinical hold on stem cell research to clear Advanced Cell Technology’s (ACT) Investigational New Drug application and initiate a Phase 1/2 clinical trial, using retinal cells derived from human embryonic stem cells (hESC) to treat patients with Stargardt disease. On January 3, 2011, ACT received FDA clearance for another new clinical trial using hESCs to treat dry AMD.
To date, the FDA has not yet cleared/approved stem cell research using adult/autologous iPSCs for clinical trials for eye disease, including dry AMD; thus, the Cellular Dynamics stem cell product will require FDA approval of its Investigational New Drug application before it can proceed into clinical trials.
In September 2014, researchers in Kobe, Japan used iPSC techniques and technology to transplant retinal cells, developed from a 70-year-old woman’s skin, into one of her eyes. According to the Wall Street Journal blog, Japanese researchers stress that “the current study is a type of pretrial research under Japanese regulations that can’t immediately lead to approved therapies or commercialization.”
Although adult/iPSC research does not use human embryos and thus sidesteps the ethical issues surrounding their use, there are still significant risks associated with the use of autologous stem cells, including tumor formation.
About Dry Macular Degeneration
The dry (also called atrophic) type of AMD affects approximately 80-90% of individuals with AMD. Its cause is unknown, it tends to progress more slowly than the wet type, and there is not – as of yet – an approved treatment or cure. “Atrophy” refers to the degeneration of cells in a portion of the body; in this case, the cell degeneration occurs in the retina.
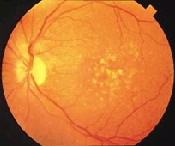
In dry age-related macular degeneration, small white or yellowish deposits, called drusen, form on the retina, in the macula – the small sensitive area in the center of the retina that provides clear central vision – causing it to deteriorate or degenerate over time.
A retina with drusen
Drusen are the hallmark of dry AMD. These small yellow deposits beneath the retina are a buildup of waste materials, composed of cholesterol, protein, and fats. Typically, when drusen first form, they do not cause vision loss. However, they are a risk factor for progressing to vision loss.
More about the Cellular Dynamics Announcement
From Cellular Dynamics International awarded National Eye Institute contract, via the Journal-Sentinel:
Cellular Dynamics International Inc. (CDI), a … maker of human cells in industrial quantities, said … it has received a $1.2 million contract from the National Eye Institute (NEI) – the company’s first agreement to manufacture its cells for therapeutic use.
Under the contract, CDI would take blood or tissue from 10 patients with [dry age-related macular degeneration], turn some of it into stem cells, then further program those cells to become retina cells.
NEI researchers plan to use these cells as part of their pre-clinical process to develop the first autologous cell transplantation treatment for dry AMD. The cells are expected to be injected in about three years into the 10 patients in the trial.
The process, known as autologous cellular therapy, would likely be the first of its kind in the U.S., CDI said. Since the cells would genetically match the patient, the expectation is that the risk of transplant rejection would be reduced.
Kapil Bharti, Ph.D., a Stadtman Investigator in the Unit on Ocular and Stem Cell Translational Research at NEI, will be the lead researcher on the stem cell project. VisionAware will provide updates on this emerging stem cell research as they become available.