Many readers have been following closely the development of Squalamine Eye Drops for wet age-related macular degeneration, hoping that a self-administered at-home eye drop could reduce, or even eliminate, the need for monthly or as-needed eye injections.
Unfortunately, a clinical trial designed to test this concept has produced disappointing results: Squalamine Eye Drops failed to reduce the average number of Lucentis injections required by the trial participants.
However, that clinical trial also revealed that squalamine produced positive changes in visual acuity – both overall in the Lucentis/squalamine treatment group, and, more significantly, in participants with a specific type of AMD lesion. As a result, a newer clinical trial, initiated in 2016 and continuing into 2019, will continue to study the effects of Squalamine Eye Drops, in combination with Lucentis injections, on gains in visual acuity in persons with wet macular degeneration.
What Is Squalamine?
Ohr Pharmaceutical, Inc. is dedicated to the clinical development of new drugs for underserved therapeutic needs. One of its lead drugs in development is Squalamine Eye Drops for the treatment of wet age-related macular degeneration (AMD).
Squalamine is a water-soluble molecule derived from the internal organs (primarily the liver) of the dogfish shark. It is believed to have great potential for treating some human viruses. Medically, squalamine has been shown to interrupt and reverse the process of angiogenesis.
About Angiogenesis and Anti-Angiogenic Drugs
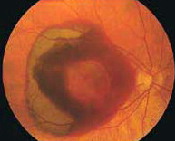
Angiogenesis is a term that describes the growth of new blood vessels and plays a critical role in the normal development of body organs and tissue. Sometimes, however, excessive and abnormal blood vessel development can occur in diseases such as cancer (tumor growth) and AMD (retinal and macular bleeding).
Substances that stop the growth of these excessive blood vessels are called anti-angiogenic (anti=against; angio=vessel; genic=development), and anti-neovascular (anti=against; neo=new; vascular=blood vessels).
The focus of current anti-angiogenic drug treatments for wet macular degeneration is to reduce the level of a particular protein (vascular endothelial growth factor, or VEGF) that stimulates abnormal blood vessel growth in the retina and macula; thus, these drugs are classified as anti-VEGF treatments.
The squalamine researchers theorized that the ability to self-administer eye drops to treat wet AMD could be more effective (and less invasive) than the current standard of care, which involves regular injections of Lucentis, Avastin, or Eylea directly into the eye, via a very small needle. These injections are administered at the doctor’s office.
A History of Squalamine Eye Drop Research
Initially, the Genaera Corporation developed the squalamine project and administered squalamine as an intravenous formulation for wet AMD in Phase 1 and Phase 2 clinical trials. However, in 2007, Genaera discontinued their clinical trials, due to financial and subject-recruitment issues. The squalamine project was then acquired by Ohr Pharmaceutical, which continued the development of squalamine in a topical eye drop formulation.
In 2012, Ohr was awarded “Fast Track” designation by the United States Food and Drug Administration (FDA) to further develop its Squalamine Eye Drop product for the potential treatment of wet AMD. Fast Track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious diseases and fill an unmet medical need. Filling an unmet medical need is defined as providing a therapy where none exists or providing a therapy that may be potentially superior to existing therapy.
The Phase 2 Clinical Trial
In August 2012, the FDA commenced a Phase 2 clinical trial to study the safety and effectiveness of Squalamine Eye Drops in combination with Lucentis injections. The researchers were seeking to determine (a) if squalamine was safe (safety) and (b) if Squalamine Eye Drops could reduce the number and frequency of Lucentis injections required to control the neovascularization and retinal bleeding associated with wet AMD (effectiveness).
The initial enrollment in the clinical trial was 142 “treatment-naïve” participants [i.e., meaning that no participant had received prior treatments] at 23 clinical ophthalmology centers across the United States. 128 participants completed the study.
All participants in the nine-month study received an initial injection of Lucentis to begin and then were randomly assigned to receive either Squalamine Eye Drops [i.e., the treatment group] or placebo eye drops twice a day throughout the study. [Editor’s note: A placebo is an inactive substance that has no therapeutic effect, used as a control in testing new drugs.]
All participants were seen monthly during the study period and received eye examinations, visual acuity testing, and optical coherence tomography (OCT) testing. Additional Lucentis injections were given on an as-needed basis to control the new blood vessel development and retinal bleeding associated with wet AMD. [Editor’s note: Optical coherence tomography (OCT) is a type of medical imaging technology that produces high-resolution cross-sectional and three-dimensional images of the eye. It is used to gain a clearer picture of the retina and its supporting layers.]
You can learn more about the Phase 2 squalamine clinical trial, including criteria for enrollment and the locations of the clinical trial study centers, at Efficacy and Safety of Squalamine Eye Drops at ClinicalTrials.gov.
The Clinical Trial Results
Safety: Squalamine Eye Drops were well-tolerated. Only two participants in the treatment group discontinued the study, due to eye pain or swelling.
Effectiveness: Unfortunately, Squalamine Eye Drops failed to decrease the average number of Lucentis injections required by the study participants. This was the primary goal of the clinical trial and the result was disappointing, both to researchers and to people with wet AMD, who were hoping that Squalamine Eye Drops could possibly reduce, or even eliminate, the need for eye injections.
There Were Some Positive Results from the Phase 2 Clinical Trial
Despite not reaching the study’s primary end goal, there were additional results from the Phase 2 clinical trial that may be encouraging. Although Squalamine Eye Drops did not reduce the need for, or the frequency of, eye injections, squalamine did produce positive changes in visual acuity, or clearness of vision – both overall in the Lucentis/squalamine treatment group, and more significantly, in participants with a specific type of AMD lesion.
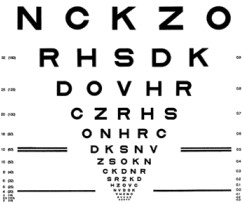
31% of the study participants who received the combined Lucentis/squalamine treatment gained 3 or more letters on the Early Treatment of Diabetic Retinopathy Study (ETDRS) Eye Chart (pictured at left), versus 25% of participants who received Lucentis treatment alone (meaning the Lucentis/placebo treatment).
However, 37 participants with “classic” AMD lesions who received the combined Lucentis/squalamine treatment gained 11 letters on the ETDRS Eye Chart, versus 5 letters with Lucentis treatment alone. Classic AMD lesions have also shown benefit in other studies of combined treatment.
A Phase 3 Clinical Trial Is Now Underway
Update: On February 15, 2107, Ohr Pharmaceuticals announced that it had temporarily suspended new enrollment in the Phase 3 clinical trial. While the 200 patients currently enrolled in the study will continue to receive the therapy, the trial will be closed for new patients.
Due to these gains in vision from the study’s combination therapy, Ohr has moved forward with a Phase 3 clinical trial. The two-year trial, which commenced in 2016, will enroll 650 participants and is estimated to be completed in mid-2019. It will continue to study the effects of Squalamine Eye Drops in combination with Lucentis injections, versus Lucentis injections alone, on best-corrected visual acuity.
“This approach is intended to provide prospective efficacy data before year end 2017 to enable us to potentially confirm the visual acuity benefits observed in the patient population we identified as the most likely to benefit from Squalamine combination therapy. Given the recent study readouts from other combination therapy agents and the reaction to these results, we feel that a change in our clinical development program is warranted,” said Dr. Jason Slakter, Chief Executive Officer of Ohr.