
Research in Vision &
Ophthalmology
Currently, there are a number of treatments for wet age-related macular degeneration, including the drugs Lucentis, Eylea, and Avastin, administered by injection with a very small needle directly into the eye after the surface has been numbed (also called “intra-vitreous injection”).
From a patient’s point of view, however, the “gold standard” for treatment would be a self-administered eye drop that replicates the function of these injectable drugs. Unfortunately, past eye drop research has proven to be unsuccessful or inconclusive:
- Squalamine eye drops, in combination with Lucentis injections, produced disappointing clinical trial results and failed to reduce the average number of Lucentis injections required by the clinical trial participants.
- Application of an eye drop compound called PPADS has not progressed from the initial “proof of concept” results reported in 2014.
This month, however, a research team from the United Kingdom reports the development of yet another novel method for delivering Lucentis and Avastin as an eye drop instead of an injectable drug, using cell-penetrating peptides (explained below). Please note that this “proof of concept” research is in its earliest stages and has been conducted only with cultured human eye cells, and rat, pig, and mouse models. It has not yet advanced into human trials. The concept of a possible topical/eye drop treatment may yet show promise for persons with wet macular degeneration.
From Investigative Ophthalmology & Visual Science
This new eye drop/macular degeneration research, titled Topical Delivery of Anti-VEGF Drugs to the Ocular Posterior Segment Using Cell-Penetrating Peptides (explained below), has been published as an open-source article in the May 2017 issue of Investigative Ophthalmology & Visual Science, the official journal of the Association for Research in Vision and Ophthalmology (ARVO). ARVO is an international organization that encourages and assists research, training, publication, and dissemination of knowledge in vision and ophthalmology, including low vision.
The authors are Felicity de Cogan; Lisa J. Hill; Aisling Lynch; Peter J. Morgan-Warren; Judith Lechner; Matthew R. Berwick; Anna F. A. Peacock; Mei Chen; Robert A. H. Scott; Heping Xu; and Ann Logan, from the University of Birmingham, United Kingdom; Queen’s University Belfast, United Kingdom; and Moorfields Eye Hospital Dubai, United Arab Emirates.
First, Some Terminology Used in the Research
Here is a brief explanation of some key terms used in this macular degeneration research:
- Posterior segment of the eyeball: The back 2/3 of the eye that includes the vitreous, retina, choroid, and optic nerve.
- Cell-penetrating peptides (CPPs): Peptides are fundamental components of cells that carry out important biological functions, including regulating the activities of other molecules. Cell-penetrating peptides (CPPs) are peptides that are able to cross, or penetrate, cell membranes and gain access to the interior of almost any cell. Because of this characteristic, CPPs are considered promising candidates for the transport of drugs or other therapeutic substances to the interior of cells, including the cells in the eye and retina.
- In vitro: Refers to processes taking place in a test tube or culture dish, typically in a laboratory. In vivo refers to processes taking place in a living human or other organism.
- Vector: A “carrier” molecule that transports drugs or therapeutic substances into another cell, where they can be expressed.
- Angiogenesis: Describes the growth of new blood vessels and plays a crucial role in the normal development of body organs and tissue. However, excessive and abnormal blood vessel development can also occur in diseases such as cancer (tumor growth), macular degeneration, and diabetic retinopathy (retinal and macular bleeding).
- Neovascularization: When referring to the eye, as in diabetic retinopathy and wet age-related macular degeneration, it describes abnormal blood vessel growth in the retina (neo = new; vascular = blood vessels). Neovascularization is also a feature of other eye and health conditions, including retinopathy of prematurity and cancer.
- VEGF: A protein, called vascular endothelial growth factor, that stimulates abnormal blood vessel growth in the retina and macula.
About the Research
Excerpted from Scientists Develop eye Drops to Treat Age-Related Blindness, via Medical Xpress:
Scientists at the University of Birmingham have developed a type of eye drop which could potentially revolutionize the treatment of one of the leading causes of blindness. The results of the collaborative research could spell the end of painful injections directly into the eye to treat the increasingly common eye disorder known age-related macular degeneration (AMD).
AMD is currently treated by repeated injections into the eye on a monthly basis over at least three years. This is a problem because, apart from being an unpleasant procedure for patients to undergo, the injections can cause tearing and infections inside the eye and an increased risk of blindness.
Now scientists led by [co-author] Dr. Felicity de Cogan, from the University of Birmingham’s Institute of Inflammation and Aging, have invented a method of delivering the injected drug as an eye drop instead, and their laboratory research has obtained the same outcomes as the injected drug.
The drop uses a cell-penetrating peptide (CPP) to deliver the drug to the relevant part of the eye within minutes. Dr. de Cogan said, “The CPP-drug has the potential to have a significant impact on the treatment of AMD by revolutionizing drug-delivery options. Efficacious self-administered drug application by eye drop would lead to a significant reduction in adverse outcomes and health care costs compared with current treatments. The CPP-plus drug complex also has potential application to other chronic ocular diseases that require drug delivery to the posterior chamber of the eye.”
More about Wet Age-Related Macular Degeneration

Age-related macular degeneration (AMD) is a gradual, progressive, painless deterioration of the macula, the small sensitive area in the center of the retina that provides clear central vision. Damage to the macula impairs the central (or “detail”) vision that helps with essential everyday activities, such as reading and writing, preparing meals, watching television, and personal self-care.
AMD is the leading cause of vision loss for people aged 60 and older in the United States. According to the American Academy of Ophthalmology, 10-15 million individuals have AMD and about 10% of people who are affected have the “wet” type of AMD.
Wet (Neovascular) Macular Degeneration
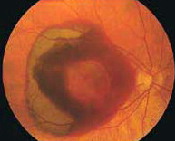
In wet macular degeneration, the choroid (a part of the eye containing blood vessels that nourish the retina) begins to sprout abnormal blood vessels that develop into a cluster under the macula, called choroidal neovascularization (neo = new; vascular = blood vessels).
The macula is the part of the retina that provides the clearest central vision. Because these new blood vessels are abnormal, they tend to break, bleed, and leak fluid under the macula, causing it to lift up and pull away from its base. This damages the fragile photoreceptor cells, which sense and receive light, resulting in a rapid and severe loss of central vision.
Eylea, Lucentis, Avastin, and Anti-Angiogenic Drugs
Angiogenesis is a term used to describe the growth of new blood vessels and plays a crucial role in the normal development of body organs and tissue.
Sometimes, however, excessive and abnormal blood vessel development can occur in diseases such as cancer (tumor growth) and AMD (retinal and macular bleeding).
Substances that stop the growth of these excessive blood vessels are called anti-angiogenic (anti=against; angio=vessel; genic=development), and anti-neovascular (anti=against; neo=new; vascular=blood vessels).
The focus of current anti-angiogenic drug treatments for wet AMD is to reduce the level of a particular protein (vascular endothelial growth factor, or VEGF) that stimulates abnormal blood vessel growth in the retina and macula; thus, these drugs, including Lucentis, Avastin, and Eylea, are classified as anti-VEGF treatments. These drugs are administered by injection with a very small needle directly into the eye after the surface has been numbed.
More About the Study from Investigative Ophthalmology & Visual Science
Excerpted from the study Introduction, Discussion, and Conclusions, with the full article available online:
The aim of this study was to assess the potential for cell-penetrating peptides (CPPs) as “chaperones” within a topical ocular drug-delivery platform for the passage of therapeutic titers [i.e., the therapeutic concentration of a solution] of VEGF antagonists [i.e., inhibitors] to the posterior segment of the eye. Use of novel CPPs as ocular drug-delivery agents would facilitate clinical administration of large biopharmaceuticals, such as ranibizumab [i.e., Lucentis] or bevacizumab [i.e., Avastin], in the form of an eye drop.
The development of this eye drop delivery platform will have wide-reaching implications for improved patient care, by reducing the side effects and treatment costs associated with both current clinically effective drugs and novel candidate drugs.
Here, we have evaluated CPP-mediated eye drop delivery of anti-VEGF antibodies in rodent and porcine [i.e., pig] models to determine dose delivery and pharmacokinetics [i.e., the movement of drugs through the body]. We also have used an established “in vivo” rodent model of choroidal neovascularization (CNV) to compare the efficacy of anti-VEGF drugs when delivered topically with CPPs or by intravitreal injection.
Anti-VEGF agents are a well-established treatment for [wet AMD]; however, the side effects from delivery by intravitreal injection represent a significant problem. Accordingly, this study investigated the use of CPP as a novel topical delivery vehicle for anti-VEGF agents to the posterior segment, negating the need for invasive intravitreal injections.
This study demonstrates that CPP can successfully deliver topically applied [i.e., eye drop] anti-VEGF drugs into mouse, rat, and pig eyes, and showed bioactivity of the topically delivered drug in a relevant disease model that was equivalent to other AMD drug-delivery methods.
Specifically, we have shown that CPPs have high penetrating capabilities for biological barriers in the eye with low toxicity and can deliver clinically relevant concentrations of anti-VEGF drugs, such as ranibizumab [i.e., Lucentis] or bevacizumab [i.e., Avastin], to the posterior segment of the eye.
In particular, these CPPs have the capacity to noninvasively deliver therapeutics … to anterior and posterior ocular segments to give outcomes comparable to … injected drugs.
If the results were translated to human eyes, it would allow the topical delivery of a wide range of ocular drugs that currently can be delivered only by intravitreal or subconjunctival injections. This would reduce treatment costs, time in clinic, and harmful side effects, while allowing patient self-administration, so that new drug-delivery regimens can be better tolerated.
VisionAware will continue report on this macular degeneration research as results become available.
Additional Information About Macular Degeneration
- Learn more about gene therapy at the VisionAware blog.
- New Macular Degeneration Research: Will My AMD Affect Both Eyes? If So, How Soon Will That Happen?
- Clinical Trial Update: Squalamine Eye Drops for Wet Macular Degeneration
- Clinical Trial Update: An Unsuccessful Trial of Combination Drugs Fovista and Lucentis for Macular Degeneration