Some good news for individuals who have diabetes and associated diabetic macular edema: On July 29, 2014, the United States Food and Drug Administration (FDA) approved EYLEA (generic name aflibercept) for the treatment of diabetic macular edema. The recommended dosage is two milligrams (mg) every two months, after five initial monthly injections.
About Diabetic Macular Edema
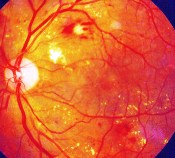
Diabetic macular edema [edema = a swelling or accumulation of fluid] (DME) can occur in people with diabetes when retinal blood vessels begin to leak into the macula, the part of the eye responsible for detailed central vision. These leakages cause the macula to thicken and swell, which, in turn, creates a progressive distortion of central vision.
Although this swelling does not always lead to severe vision loss or blindness, it can cause a significant loss of central, or detail, vision, and is the primary cause of vision loss in people with diabetic retinopathy.
A Short History of the Development of EYLEA
EYLEA is an anti-angiogenic drug (more about that below) that was developed for injection in the eye to block blood vessel growth in age-related macular degeneration (AMD).
In February 2011, Regeneron Pharmaceuticals, Inc. announced that the company had submitted an application to the FDA for Regeneron’s VEGF Trap-Eye (now called EYLEA™), a potential injectable drug treatment for wet AMD.
Regeneron’s VEGF Trap-Eye application to the FDA was based on positive results from two Phase III clinical trials: the North American VIEW 1 trial and the global VIEW 2 trial.
The VIEW (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD) program consists of two randomized, double-blind, Phase III clinical trials.
The VIEW 1 study, conducted in the United States and Canada, included 1,217 patients. The VIEW 2 study, conducted in Europe, Asia Pacific, Japan, and Latin America, included 1,240 patients.
At the conclusion of both clinical trials, the FDA approved EYLEA on November 18, 2011. Although the FDA has ruled that EYLEA’s benefits outweigh its risks, the drug can cause a number of side effects, including bleeding at the injection site, eye pain, cataracts, floaters, and elevated eye pressure.
Clinical Trials Supporting the Current EYLEA FDA Approval for Diabetic Macular Edema
The safety and effectiveness of EYLEA in treating diabetic macular edema were established via two ongoing clinical trials: the VISTA-DME trial and the VIVID-DME trial.
The purpose of these clinical trials is to determine the effectiveness of “VEGF Trap-Eye,” administered by injection, on best-corrected visual acuity as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) eye chart in subjects with diabetic macular edema with central visual field involvement.
The ETDRS Eye Chart
The 461 subjects in the VISTA-DME trial were randomly assigned to one of the following three groups: (1) EYLEA given monthly (n=155); (2) EYLEA given every two months (n=152); or (3) the control/comparison treatment of laser photocoagulation (n=154).
The 404 subjects in the VIVID-DME trial were randomly assigned to one of the following three groups: (1) EYLEA given monthly (n=136); (2) EYLEA given every two months (n=135); or (3) the control/comparison treatment of laser photocoagulation (n=132).
More about the Current FDA Approval for Diabetic Macular Edema
From FDA approves EYLEA Injection for treatment of Diabetic Macular Edema at Medical News:
Regeneron Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration (FDA) has approved EYLEA® (aflibercept) Injection for the treatment of Diabetic Macular Edema (DME). The recommended dosage of EYLEA in patients with DME is 2 milligrams (mg) every two months (8 weeks) after five initial monthly injections. Although EYLEA may be dosed as frequently as 2 mg every 4 weeks, additional efficacy was not demonstrated when EYLEA was dosed every 4 weeks compared to every 8 weeks.
The approval of EYLEA in DME was based on the one-year data from the Phase 3 VISTA-DME and VIVID-DME studies of 862 patients, which compared (a) EYLEA 2 mg given monthly, (b) EYLEA 2 mg given every two months (after five initial monthly injections), or (c) macular laser photocoagulation (at baseline and then as needed).
In the DME studies, after one year, the mean changes in Best Corrected Visual Acuity (BCVA), as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) chart for the monthly and every two month EYLEA groups, were statistically significantly improved compared to the control group and were similar to each other.
Across both trials, patients in both EYLEA dosing groups gained, on average, the ability to read approximately two additional lines on an eye chart compared with almost no change in the control group.
EYLEA and Anti-Angiogenic Drugs
Angiogenesis is a term used to describe the growth of new blood vessels and plays a crucial role in the normal development of body organs and tissue. Sometimes, however, excessive and abnormal blood vessel development can occur in diseases such as cancer (tumor growth) and AMD (retinal and macular bleeding).
Substances that stop the growth of these excessive blood vessels are called anti-angiogenic (anti=against; angio=vessel; genic=development), and anti-neovascular (anti=against; neo=new; vascular=blood vessels).
The focus of current anti-angiogenic drug treatments is to reduce the level of a particular protein (vascular endothelial growth factor, or VEGF) that stimulates abnormal blood vessel growth in the retina and macula; thus, these drugs are classified as anti-VEGF treatments.
At present, these drugs are administered by injection directly into the eye after the surface has been numbed. Current anti-VEGF drugs in use include EYLEA, Avastin, and Lucentis.