
Currently, there are a number of treatments for wet age-related macular degeneration, including the drugs Lucentis, Eylea, and Avastin, administered by injection with a very small needle directly into the eye after the surface has been numbed (also called “intra-vitreous injection”).
There are also a number of treatments that have proven to be inconclusive or unsuccessful after undergoing clinical trials, including stem cells, eye drops, and combination drug treatments.
Most recently, a research team led by Johns Hopkins University School of Medicine is reporting a potentially new approach to the treatment of wet age-related macular degeneration, using gene therapy.
In their research, a virus similar to a cold, but modified in the lab to prevent it from causing disease, is used as a gene carrier and injected into the human eye. This virus, called AAV2, deposits a gene that causes the retinal cells to produce a therapeutic protein called sFLT01, which can halt the creation of abnormal blood vessels.
Please note: Although this very early stage gene therapy research is reported as being safe for humans in a preliminary clinical trial, the research team also states that it may have serious limitations for broader use, due to a built-in immunity to the AAV2 virus in a significant portion of the United States population, as explained below.
From The Lancet
This new macular degeneration gene research, titled Intravitreous injection of AAV2-sFLT01 (explained below) in patients with advanced neovascular [i.e., wet] age-related macular degeneration, has been published in the May 16, 2017 edition of The Lancet.
The Lancet, which has been published continuously for 180 years, is one of the world’s leading independent medical journals, without affiliation to a medical or scientific organization. The journal, which is committed to international health concerns, publishes high-quality clinical trials that influence the course of medical practice.
The authors are Jeffrey S Heier, MD; Saleema Kherani, MD; Shilpa Desai, MD; Pravin Dugel, MD; Shalesh Kaushal, MD; Seng H Cheng, PhD; Cheryl Delacono, OD; Annie Purvis, MSPH; Susan Richards, PhD; Annaig Le-Halpere, PharmD; John Connelly, MBA; Samuel C Wadsworth, PhD; Rafael Varona, MD; Ronald Buggage, MD; Abraham Scaria, PhD; and Peter A. Campochiaro, MD, from Johns Hopkins University School of Medicine, Baltimore MD; Ophthalmic Consultants of Boston, Boston, MA; Retinal Consultants of Arizona, Phoenix; University of Massachusetts Medical Center, Worcester, MA; and Sanofi Genzyme, Cambridge, MA.
First, Some Terminology Used in the Research
Here is a brief explanation of some key terms used in this macular degeneration gene research:
- Gene therapy: A delivery system for drugs. It is a treatment in which genetic material is introduced into cells, either to compensate for an abnormal gene or to create a therapeutic protein, such as AAV2-sFLT01, used in this research.
- Vector: A “carrier” molecule that transports genetic material into another cell, where it can be reproduced and/or expressed.
- Adeno-associated virus, or AAV: A virus that infects humans and some other primate species. AAV does not cause disease; instead, it causes a mild immune response.
- AAV2: A variation of AAV. It is similar to the common cold, altered in the lab so that it does not cause disease. It is used as a carrier, or vector, for a gene and is injected into the eye. The AAV2 virus penetrates retinal cells and deposits the gene, which causes the retinal cells to produce a therapeutic protein called sFLT01.
- sFLT01: A protein that diminishes the growth of abnormal blood vessels under the retina.
- Angiogenesis: Describes the growth of new blood vessels and plays a crucial role in the normal development of body organs and tissue. However, excessive and abnormal blood vessel development can also occur in diseases such as cancer (tumor growth), macular degeneration, and diabetic retinopathy.
- Neovascularization: When referring to the eye, as in wet age-related macular degeneration, it describes abnormal blood vessel growth in the retina (neo = new; vascular = blood vessels).
- VEGF: A protein, called vascular endothelial growth factor, that stimulates abnormal blood vessel growth in the retina and macula.
About the Research
Excerpted from New Gene Therapy for Vision Loss Proven Safe in Humans, via Johns Hopkins Medicine:
In a small and preliminary clinical trial, Johns Hopkins researchers and their collaborators have shown that an experimental gene therapy that uses viruses to introduce a therapeutic gene into the eye is safe and that it may be effective in preserving the vision of people with wet age-related macular degeneration (AMD).
The study reports an approach in which a virus, AAV2, which is similar to the common cold but altered in the lab so that it is unable to cause disease, is used as a carrier for a gene and is injected into the eye. The virus penetrates retinal cells and deposits the gene, which turns the cells into factories for productions of a therapeutic protein, called sFLT01.
The phase 1 clinical trial involved 19 men and women, 50 years old or older with advanced wet AMD.
Participants were divided into five different groups that received increasing doses. Each group was examined by investigators for signs of adverse reactions for at least four weeks before administering a higher dose to the next group.
After the virus deposited the gene, the cells began secreting sFLT01 which bound to VEGF and prevented it from stimulating leakage and growth of abnormal blood vessels. The goal is for the retinal cells infected by the virus to produce enough sFLT01 to permanently stop the progression of AMD.
After monitoring the first three groups and finding no dose-limiting toxicity, the researchers administered the maximum dose to a group of ten participants and observed no serious side effects. “Even at the highest dose, the treatment was quite safe. We found there were almost no adverse reactions in our patients,” [Study co-author] Peter Campochiaro, MD, says.
[However], five participants showed no reduction in [retinal] fluid levels. Surprisingly, the researchers say, they found that all of the patients who did not show improvement had pre-existing antibodies to the AAV2 virus.
From that result, the researchers conclude that even if further studies affirm the safety and value of their gene therapy, it may have limitations for broad use. That’s because an estimated sixty percent of the U.S. population has been infected with adeno-associated virus, the family of viruses that AAV2 belongs to, and have built an immunity to it. The researchers believe that in these patients, the immune system destroyed the virus before it could insert the therapeutic gene.
Dr. Campochiaro explains, “The numbers are small and simply show a correlation, so we don’t know if serum antibodies are definitely an impediment, but more work is needed to determine this.”
More about Wet Age-Related Macular Degeneration

Age-related macular degeneration (AMD) is a gradual, progressive, painless deterioration of the macula, the small sensitive area in the center of the retina that provides clear central vision. Damage to the macula impairs the central (or “detail”) vision that helps with essential everyday activities, such as reading and writing, preparing meals, watching television, and personal self-care.
AMD is the leading cause of vision loss for people aged 60 and older in the United States. According to the American Academy of Ophthalmology, 10-15 million individuals have AMD and about 10% of people who are affected have the “wet” type of AMD.
Wet (Neovascular) Macular Degeneration
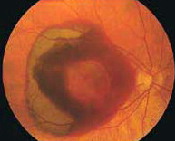
In wet macular degeneration, the choroid (a part of the eye containing blood vessels that nourish the retina) begins to sprout abnormal blood vessels that develop into a cluster under the macula, called choroidal neovascularization (neo = new; vascular = blood vessels).
The macula is the part of the retina that provides the clearest central vision. Because these new blood vessels are abnormal, they tend to break, bleed, and leak fluid under the macula, causing it to lift up and pull away from its base. This damages the fragile photoreceptor cells, which sense and receive light, resulting in a rapid and severe loss of central vision.
Eylea, Lucentis, Avastin, and Anti-Angiogenic Drugs
Angiogenesis is a term used to describe the growth of new blood vessels and plays a crucial role in the normal development of body organs and tissue.
Sometimes, however, excessive and abnormal blood vessel development can occur in diseases such as cancer (tumor growth) and AMD (retinal and macular bleeding).
Substances that stop the growth of these excessive blood vessels are called anti-angiogenic (anti=against; angio=vessel; genic=development), and anti-neovascular (anti=against; neo=new; vascular=blood vessels).
The focus of current anti-angiogenic drug treatments for wet AMD is to reduce the level of a particular protein (vascular endothelial growth factor, or VEGF) that stimulates abnormal blood vessel growth in the retina and macula; thus, these drugs, including Lucentis, Avastin, and Eylea, are classified as anti-VEGF treatments. These drugs are administered by injection with a very small needle directly into the eye after the surface has been numbed.
About Clinical Trials
Most clinical trials are designated as Phase 1, 2, or 3, based on the questions the study is seeking to answer:
- In Phase 1 clinical trials, researchers test a new drug or treatment in a small group of people for the first time to evaluate its safety, determine a safe and effective dosage range, and identify possible side effects.
- In Phase 2 clinical trials, the study drug or treatment is given to a larger group of people to determine if it is effective and to further evaluate its safety.
- In Phase 3 studies, the study drug or treatment is given to even larger groups of people (1,000-3,000) to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.
- In Phase 4 studies, after the United States Food and Drug Administration (FDA) has approved the drug, continuing studies will determine additional information, such as the drug’s risks, side effects, benefits, and optimal use.
More About the Study from The Lancet
Excerpted from the study Abstract:
Background: Long-term intraocular injections of vascular endothelial growth factor (VEGF)-neutralizing proteins can preserve central vision in many patients with neovascular age-related macular degeneration. We tested the safety and tolerability of a single intravitreous injection of an AAV2 vector expressing the VEGF-neutralizing protein sFLT01 in patients with advanced neovascular age-related macular degeneration.
Methods: This was a phase 1, open-label, dose-escalating study done at four outpatient retina clinics in the USA. Patients were assigned to each cohort in order of enrollment, with the first three patients being assigned to, and completing, the first cohort before filling positions in the following treatment groups.
Patients aged 50 years or older with neovascular age-related macular degeneration and a baseline best-corrected visual acuity score of 20/100 or less in the study eye were enrolled in four dose-ranging cohorts (or groups) and one maximum tolerated dose cohort and followed up for 52 weeks.
The primary objective of the study was to assess the safety and tolerability of a single intravitreous injection of AAV2-sFLT01, through the measurement of eye-related adverse events.
Interpretation: Intravitreous injection of AAV2-sFLT01 seemed to be safe and well tolerated at all doses. Additional studies are needed to identify sources of variability in expression and anti-permeability activity, including the potential effect of baseline anti-AAV2 serum antibodies.
VisionAware will continue report on this, and other, genetic research as results become available.
Additional Information About Macular Degeneration
- Learn more about gene therapy at the VisionAware blog.
- New Macular Degeneration Research: Will My AMD Affect Both Eyes? If So, How Soon Will That Happen?
- Clinical Trial Update: Squalamine Eye Drops for Wet Macular Degeneration
- Clinical Trial Update: An Unsuccessful Trial of Combination Drugs Fovista and Lucentis for Macular Degeneration